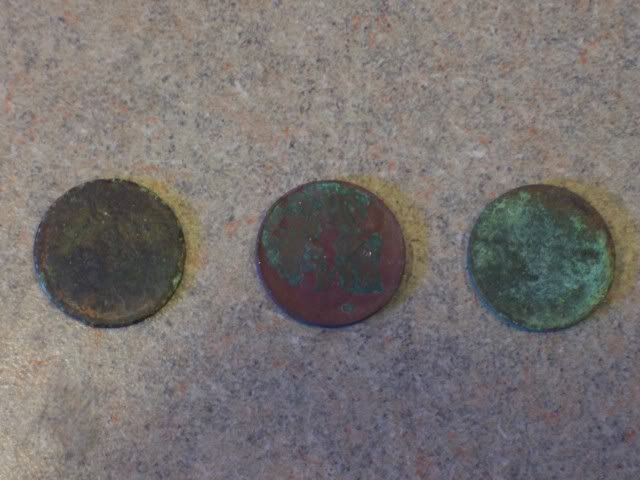Microwave Peroxide for 15 to 40 seconds tops. Drop coins in and SOAK for 15 minutes.
What I do and why: Put the coins and Peroxide together in a pyrex custard dish and then nuke them together in the microwave for about 40 seconds, by 40 seconds it is really bubbling vigoriously. Microwaving copper coins with the peroxide heats up the coins and liquid together and therefore keeps the peroxide bubbling longer, instead of dumping cold coins into hot peroxide which cools it down quicker and hense less cleaning action. Microwaves work on heating stuff by exciting dipole moments, water is a perfect dipole molecule with its two hydrogens and one oxygen. The microwaves work on the two hydrogens attached to the oxygen and gets them fluttering excitedly thus creating heat. Metal atoms also have dipole moments, along with most other compounds, but they are locked together much more tighly and take longer to get as excited as water does. So making a long story short, microwave ovens heat up food quickly because of their water. Yes, given enough time most other compounds will heat up and eventually burn in a microwave. Microwave popcorn bags have a special thin metal plate at the bottom of the bag that is designed to heat up the butter and corn. When the water in the popcorn kernel steams and causes the corn to pop it will be coated with the hot oil butter. I'm not sure what the properties of the plate are but it definately assists in the heating process, must be something with a good dipole moment, this is why the bag is so darn hot. Side note some microwaves even have metal shelves, and they work just fine. With all microwave ovens you're warned not to turn on the oven with nothing inside, this is so the waves of energy are focused on the item in the oven and not the oven itself which is made of metal and other parts that can eventually heat up or electrically arc. Likewise if you just put the coins in or a spoon with no liquid at all bad things will happen, like arcing because the energy has to be released some how. Microwaves would rather work on water than metal anyday!!
Drugstore Hydrogen peroxide is 3% H2O2 and 97% H2O. When we boil peroxide the one oxygen in H2O2 gets released as pure oxygen and leaves water as the other by-product. The pure oxygen is what reacts with the dirt, grim and light green copper (oxidized cooper) and releases them from the surface of the coin to get to the pure copper that is still in the coin. I'd have to review my chemistry a bit more but the dark brown we end up with is the newly oxidized copper. Verdigris green copper is a step further in the oxidation process I believe, and is a point where no more oxygen can get to the bare copper underneath and provides a protective barrior. This whole process of oxidation gives copper products there long life in the elements. Silver is far more resistant to oxidation and this is the reason why this technicue doesn't work as good as it does on copper. If anything it might tarnish (blacken) silver quicker than otherwise. Gold should be unaffected by this or just about anything else for that matter, and is the reason why it is always bright and lustrious forever.
I stand to be corrected and welcome any critique others might have. Disclaimer: It's your microwave so sometimes its best not to listen to fools on the internet, and do what you feel is safe thumbsup01!!